This is an old revision of the document!
Those who fail to plan...
I’ve found that the old adage of “Those who fail to plan, plan to fail”, is easily modified to state:

As a Quality Management System (QMS) auditor and consultant, I’ve visited many companies during my career… including several Fortune 500 companies. And I've observed that MANY of the nonconformities identified, whether internally or from external QMS auditors, have similar root causes. This is so common that some “Corrective Action” software databases include a selection of “typical” root causes in “Drop-Down List” - presumably to allow management to create Pareto charts for analysis! For example:
| Category | Root Cause |
|---|---|
| Personnel | Training Content (e.g., incomplete, confusing/cryptic, obsolete) |
| Training not updated to stay aligned with process changes (e.g., new equipment) | |
| Instructor Knowledge / Competence (Lack of a “Train the Trainer” program) | |
| Trainee Comprehension / Understanding (Lack of, or inadequate testing) | |
| Trainee Competence (Ineffective of demonstration of competence) | |
| Requirements not communicated (due to ineffective communication between functions & Personnel) | |
| Materials | Incorrect material received - due to incorrect specifications communicated to the supplier |
| Incorrect material received - due to inadequate control of changes to orders | |
| Incorrect material received - due to unauthorised substitution | |
| Nonconforming material received - due to inadequate material acceptance inspection requirements | |
| Nonconforming material received - due to no acceptance inspection/verification performed on material | |
| Nonconforming material - due to incorrect, incomplete, or missing identification | |
| Nonconforming material - due to improper handling packaging (damage) | |
| Nonconforming material - due to improper storage of materials (damage or spoilage) | |
| Nonconforming material - due to Material shelf life exceeded | |
| Measurements | Tolerances not clearly specified |
| Tolerances are specified, but not achievable with existing equipment | |
| Inadequate performance measurement and assessment | |
| Process Capability is unknown | |
| Equipment | Improper or Inadequate Measuring and/or Test Equipment (e.g., lacking accuracy, past its calibration due date) |
| Equipment not under a Preventive Maintenance Program | |
| Methods | No documented procedures/work instructions exists for process |
| Procedures/work instructions not readily available to personnel | |
| Procedures/work instructions do not include clear revision levels (to determine the applicable version) | |
| Inadequate recall/removals of obsolete procedures/work instructions | |
| Environment | Lighting (e.g., Lack of defined requirements for illumination) |
| Temperature and/or humidity requirements not defined for material(s) and/or process(es) | |
| Temperature and/or humidity requirements exceeded but not being monitored or reported | |
| Foreign Object Debris / Damage (FOD) |
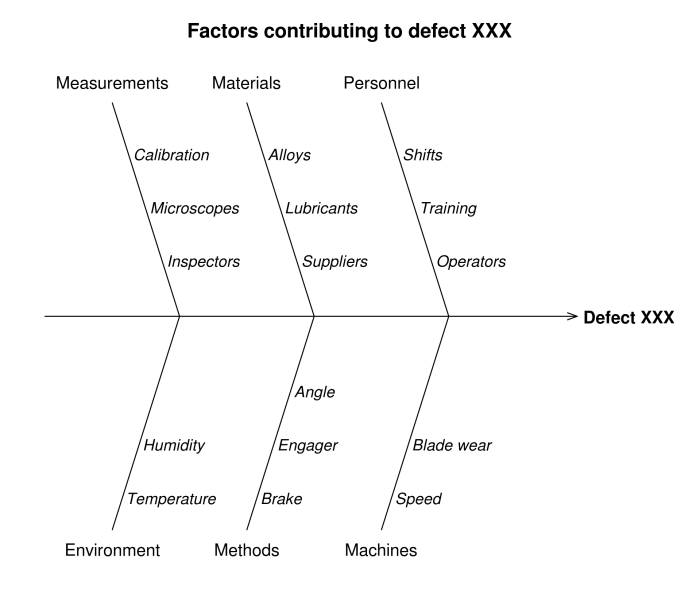
While many “Corrective Action” software companies create their own categories, I chose the above categories because they reflect the 6 Ms (Manpower, Materials, Measurements, Machines, Methods & Mother Nature) that originated with Lean Manufacturing and the Toyota Production System. These same categories typically appear in Ishikawa Fishbone charts. And Fishbone charts are commonly used as a structured approach to identifying “Failure Modes” in a FMEA (Failure Mode and Effects Analysis).
If your company is creating Pareto charts to analyze and differentiate the “significant few” from the “trivial many” root causes… you have a fundamentally flawed QMS!
Had a series of PFMEAs (Process Failure Mode and Effects Analysis) been completed during the planning stage, virtually all of the above “root causes” would have been eliminated before they were realized.
Very few of the companies I've visited have ever performed any PFMEAs as part of their planning. IF they had, then the costs associated with their product returns, customer complaints, and performing individual Root Cause Analysis (RCAs) and Corrective Actions would have been dramatically reduced.
So why isn't your company performing PFMEAs?